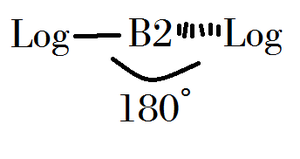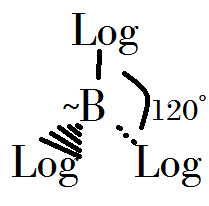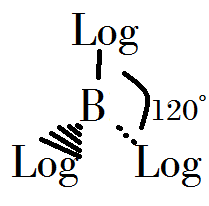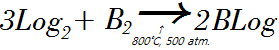Logicium
Logicium is the most boring element in the Nonsensical Table of the Elements. It also occupies the first ever spot in the table - with atomic number negative infinity.
General Properties[edit | edit source]
Logicium has a very boring property. It has negative infinity protons, in 4 isotopes. The primary isotope has positive infinity neutrons. The percentage adds up is 100%, which is boring. BORING!
Logicium is obviously known for its boredom, but it's also known for its special property, which is, if anyone drinks a small amount of logicium, that person falls asleep no matter how hard one may try. It has no color and no smell, but it is classified in the Odorful Gases because it stinks.
Usage[edit | edit source]
In medicine, Logicium is an anaesthetic gas. It is useful for physicians to induce anesthesia to patients.
Natural thingy[edit | edit source]
Logicium can be found in polybeeron dilogicium, and in materials such as Math book, air in the classroom, college papers...
Isolation[edit | edit source]
Logicium can be isolated and purified when heated to 1500˚C at 300 atm. Just like normal atoms do. How boring!
Synthesis[edit | edit source]
Logicium can be made using Negator with Illogicium. The number of neutrons is automatically decided based on which isotopes you used for making.
Organic Synthesis[edit | edit source]
Logicium can make a strong bond with Boron, Beeron and B-ron. which all are also quite boring.
- B-ron dilogicide is really toxic and dangerous. If consumed or contacted by Illogical people, severe injury (or death) can occur.
- Dilute B-ron dilogicide can be used for nullifying Illogical elements. It is most used in laboratories, but factories use them.
- Beeron trilogicide is used for people who don't sleep with pure Logicium. It's a stronger anaesthetic gases.
Laboratory Synthesis[edit | edit source]
- Boron trilogicide can be made when it's heated and compressed at 800˚C, 500atm.